Abstract
Background
Xenogenic organ replacement from pig to human is a possibility provided sterility and immunogenicity are taken care of. The objective of this study was to check the sterility of processed porcine pulmonary xenografts to mark them ‘safe’ for clinical application, without the threat for xenozoonoses.
Methods
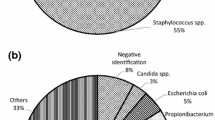
A total of 148 porcine pulmonary valve conduits were screened pre and post processing for bacterial, fungal and viral contamination. The bacterial and fungal contaminants such as Candida species, filamentous fungus, yeast like organisms, gram negative bacteria and porcine endogenous retrovirus (PERV) were isolated from the preprocessed porcine conduits. These harvested conduits were collected in a cocktail of antimicrobials and Hanks balanced salt solution. Next, they were subjected to ‘processing’ i.e., made completely acellular, strong and biocompatible in all respects. Finally, sterility checks were carried out for bacterial, fungal and viral (PERV) contamination.
Result
Microbial evaluation performed in this study revealed that the porcine pulmonary conduits were made ‘microbe free’ on subjecting to the special staged decellularization technique.
Conclusion
The porcine xenograft conduit processed in the laboratory, which has an added advantage of numbers and sizes, can replace the gold standard homograft safely.
Similar content being viewed by others
References
Sodian R, Hoerstrup SP, Sperling JS, et.al. Tissue engineering of heart valves: in vitro experiences. Ann Thorac Surg 2000; 70: 140–144.
Takeuchi Y, Magre S, Patience C. The potential hazards of xenotransplantation an over view. Rev SciTech off Int Epiz 2005; 24: 323–334.
Pitkin Z, Mullon C. Evidence of absence of poricine endogenous retrovirus (PERV) infection in patients treated with a bioartificial liver support system. Artif Organs 1999; 23: 829–833.
Ericsson TA, Takeuchi Y, Templin Cet.al. Identification of receptors for pig endogenous retrovirus. Proc Natl Acad Sci U.S.A. 2003; 100: 6759–6764.
Magre S, Takeuchi Y, Bartosch B. Xenotransplantation and pig endogenous retroviruses. Rev Med Virol 2003; 13: 311.
Martin U, Winkler ME, Id M, et al. Productive infection of primary human endothelial cells by pig endogenous retrovirus (PERV). Xenotransplantation 2000; 7: 138–142.
Carabello BA, Crawford FA. Jr Valvular heart disease. N Engl J Med 1997; 337: 32–41.
Nugent HM, Edelman ER. Tissue engineering therapy for cardiovascular disease. Circ Res 2003; 92: 1068–1078.
Bader A, Schilling T, Teebken OE, et al. Tissue engineering of heart valves — human endothelial cell seeding of detergent acellularised porcine valves. Eur J Sur 1998; 14: 279–284.
Pfaller M, Houston A, Coffmann S. Application of CHROM Agar for rapid screening of clinical specimens for Candida albicans, Candida tropicalis, Candida Krusei and Candida (torulopsis) glabrata. J Clin Microbiol 1996; 34: 58–61.
Baily W Robert and Glvyn G Scott (1994). Diagnostic Microbilogy Book, 9th edition, A text book for the isolation and identification of pathogenic microgranism. Page 66.
Myer R, Koshi G. Manual of Diagnostic procedures in Medical Microbiology and Immunology / Serology: (All India Press, Pondicherry) 1982; 50–52
Harris J L. Modified methods for fungal slide culture. J Clin Microbiol 1986; 24: 460–461.
Herring C, Quinn G, Bower R, et al. Mapping full-length porcine endogenous retroviruses in a large white pig. J Virol 2001; 75: 12252–12265.
Bartosch B, Stefanidis D, Myers R, Weiss R, Patience C, Takeuchi Y. Evidence and consequence of porcine endogenous retrovirus recombination. J Virol 2004; 78: 13880–13890.
Blusch JH, Patience C, Takeuchi Y, et al. Infection of non human primate cells by Pig endogenous retrovirus. J Virol 2000; 74: 7687–7690.
Irgang M, Karlas A, Lave C, et al. Porcine endogenous retrovirous PERV-A and PERV-B infect neither mouse cells in vitro nor SCID mice in vivo. Intervirology 2005; 48: 167–173.
Martina Y, Marcucci K T, Chergui S, et al. Mice transgenic for a human Porcine endogenous retrovirus receptor are susceptible to productive viral infection. J Virol 2006; 80: 3135–3146.
Dinsmore JH, Manhart C, Raineri R, Jacoby DB, Moore A. No evidence for infection of human cells with porcine endogenous retrovirus (PERV) after exposure to porcine fetal neuronal cells. Transplantation 2000; 70: 1382–1389.
Grauss RW, Hazekamp MG, Oppenhuizen F, et al. Histological evaluation of decellularised porcine aortic valves: matrix changes due to different decellularisation methods. Eur J Cardiothorac Surg 2005; 27: 566–571.
Fiddler GI, Gerlis LM, Walker DR, Scott O, Williams GJ. Calcification of hutaraldehyde — preserved porcine and bouine xenograft valves in young Children. Ann Thoracic Surgery 1983; 35: 257–261.
Guhathakurta S, Verghese S, Balasubramanian V, et al. Technique to process xenogenic tissues for cardiovascular implantation — A preliminary report. Current Science 2006; 91: 1068–1073.
Vyavahare N, Hirsch D, Lerner E. Prevention of bioprosthetic heart valve calcification by ethanol preincubation. Efficacy and mechanisms Circulation 1997; 95: 479–488.
Clark JN, Ogle MF, Ashworth P, Bianco RW, Levy RJ. Prevention of calcification of bioprosthetic heart valve cusp and aortic wall with ethanol and aluminium chloride. Ann Thorac Surg 2005; 79: 897–904.
Hodde J, Hiles M. Various safety of a porcine — derived medical device: Evaluation of a viral inactivation method. Biotechnol Bioeng 2002; 79: 211–216.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balasundari, R., Gupta, R., Sivasubramanian, V. et al. Complete microbe free processed porcine xenograft for clinical use. Indian J Thorac Cardiovasc Surg 23, 240–245 (2007). https://doi.org/10.1007/s12055-007-0049-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12055-007-0049-y